Dye Drop Experimental Protocol
Dye drop methods use a sequence of solutions each made slightly denser than the last by addition of iodixanol (OptiPrepTM), an inert liquid used in radiology. Denser solutions displace the previous solution with high efficiency and minimal mixing. This effectively eliminates the need for mix and wash steps, where most of the uneven cell loss occurs.
On this page, we give an overview of the dye drop and deep dye drop protocols. For more detailed protocols used to generate our published results, please visit protocols.io.
Dye Drop
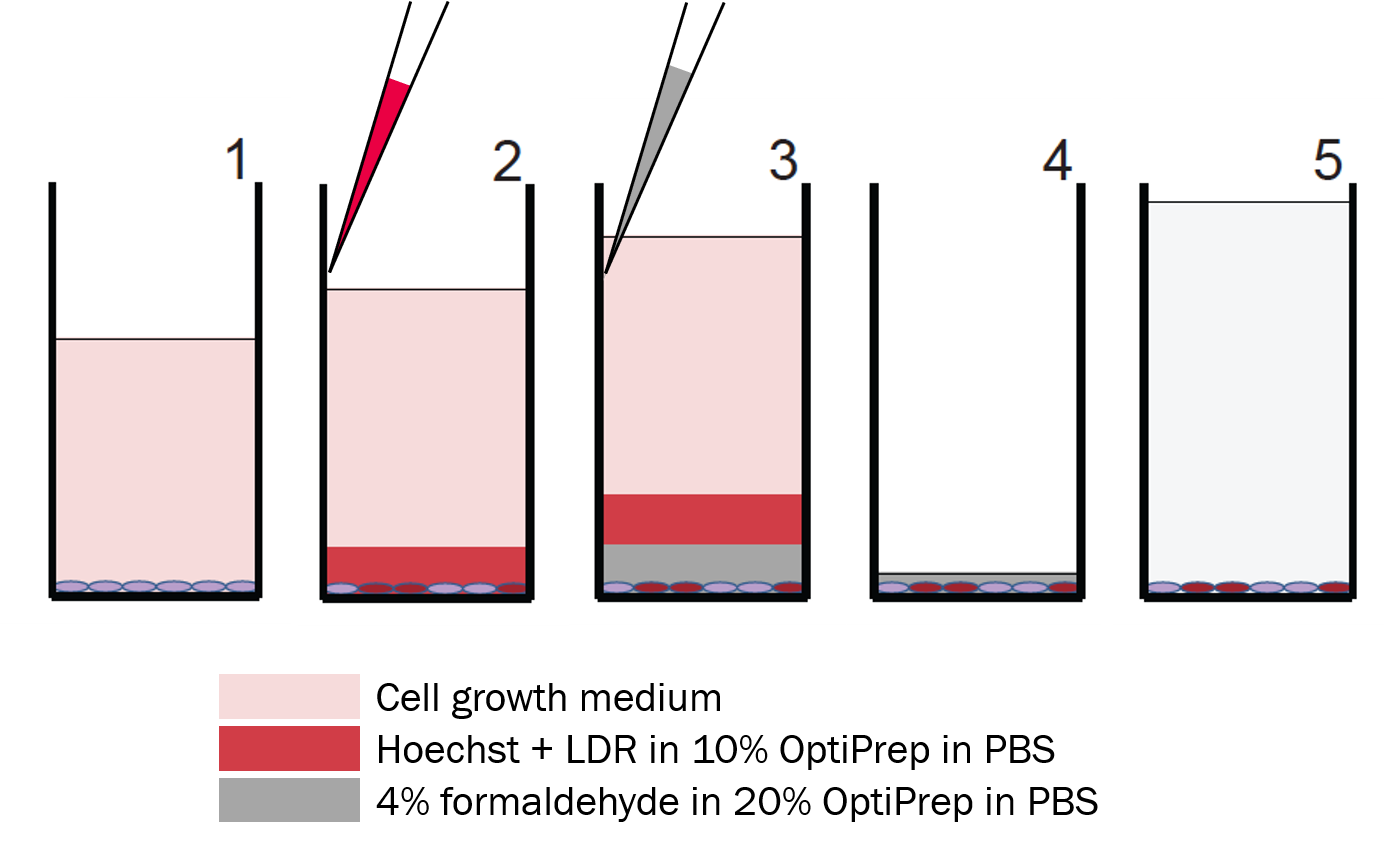
Step 1: Start with adherent cells in growth medium
Step 2: Stain
Using a multi-channel pipette, gently add Hoechst+LIVE/DEAD Far Red Fluorescent dye (LDR) in 10% OptiPrepTM in phosphate buffered saline (PBS) along the wall of the wells.
Only dead cells are stained by LDR.
Step 3: Fix
Add 4% formaldehyde in 20% OptiPrepTM in PBS along the wall of the wells. This solution will displace the stain solution at the bottom of the well.
Step 4: Aspirate
Remove the solutions in the wells, leaving some formaldehyde at the bottom to minimize cell loss. Refill the wells with PBS, seal the plates.
Step 5: Image or Store
Image plates immediately or store fixed cells in PBS at 4°C up to several weeks.
Deep Dye Drop
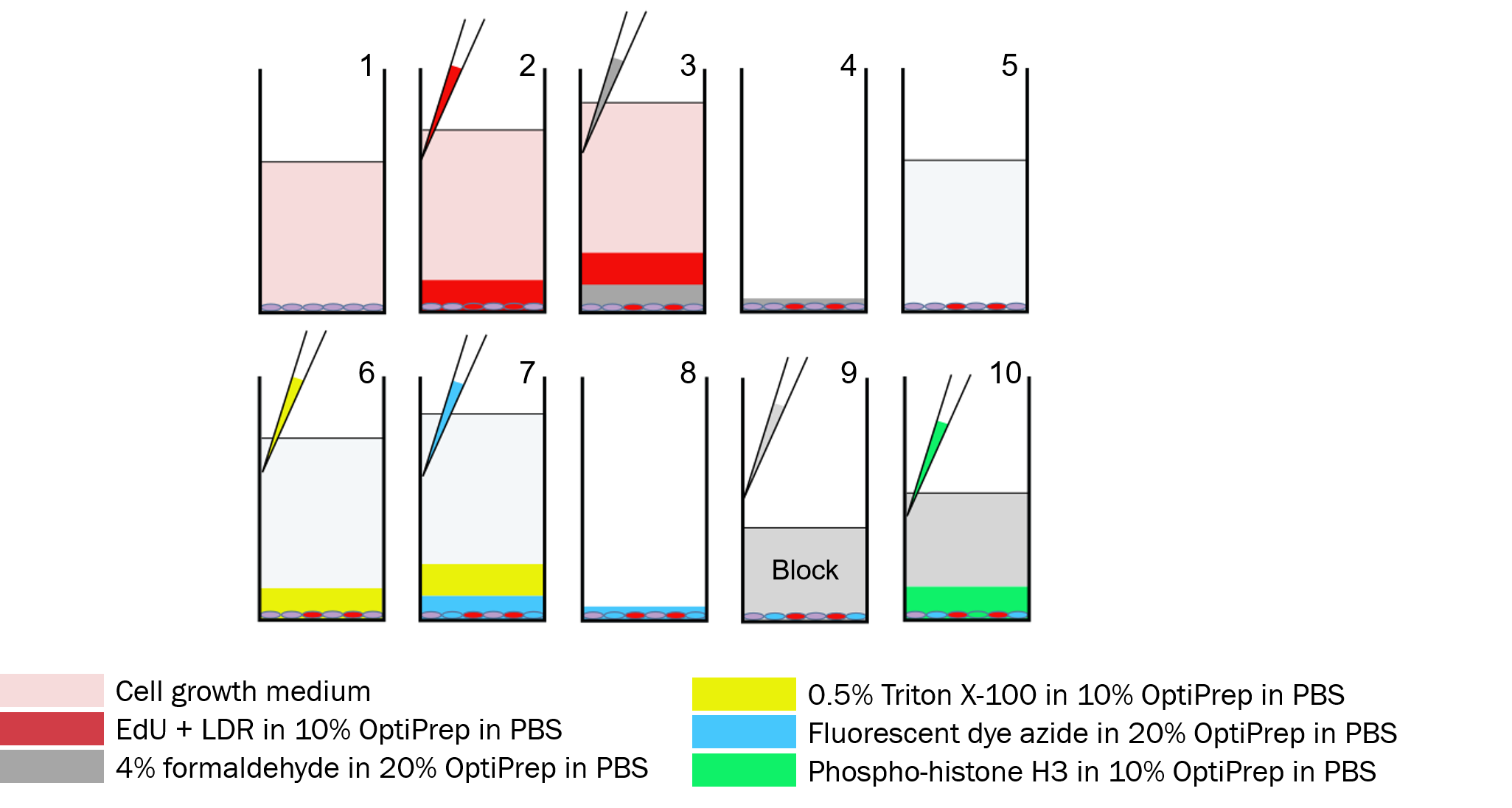
Step 1: Start with adherent cells in growth medium
Step 2: LDR + EdU Stain
Using a multi-channel pipette, gently add LIVE/DEAD Far Red FLuorescent dye (LDR) and EdU (5-ethylnyl-2’-deoxyuridine) in 10% OptiPrepTM in PBS solution along the wall of the wells.
Only dead cells are stained by LDR.
Steps 3-5: Same as Dye Drop
Store in PBS at the end of Step 5
Step 6: Permeabilize
Add 0.5% Triton X-100 in 10% OptiPrepTM to permeabilize cell membranes.
Step 7: Click Label EdU
Label EdU with a fluorescent dye azide via Click chemistry in 20% OptiPrepTM
Only S-phase cells are stained by fluorescently-labeled EdU.
Step 8: Aspirate
Remove solutions from the wells via gentle aspiration.
Step 9: Block
Add blocking solution.
Step 10: pH3 Antibody
Stain with a conjugated antibody against phospho-histone H3 (pH3) in 10% OptiPrepTM
Only M-phase cells are stained by the pH3 antibody.

